
The clinical symptoms of chronic kidney disease in cats (CKD) are often hardly noticeable for a long time. Muscle loss is a first indication that should not be neglected.
The loss of muscle is accompanied by a loss of weight. A weight loss of 5% – that would be 250 g in a 5 kg cat – is already a serious sign of a possible CKD. As weight loss increases, emaciation (cachexia) occurs, where amino acids are drawn from the muscle as a primary source of energy. This occurs when the cat does not eat enough protein and calories.
A weight loss of 5% is already a serious sign of a possible CKD.
In addition, protein digestion disorders may be present in CKD. In this case, fewer proteins can be digested and a reduced amino acid absorption from the small intestine occurs. Impaired protein digestion leads to the growth of the very bacteria in the intestine that break down amino acids into precursors of uraemic toxinsToxic, nitrogen-containing urinary substances responsible for uraemia and kidney damage.....
This underlines the need for good nutritional management in cats with chronic kidney disease.
Disturbed amino acid profile
Amino acids are needed to create proteins. There are 20 proteinogenic amino acids, 11 of which are essential for cats. Unlike non-essential amino acids, essential amino acids cannot be synthesised by the organism and must be obtained from the diet. Humans, dogs and cats with kidney disease, even those with early disease, have been reported to have an impaired amino acid profile in the blood.
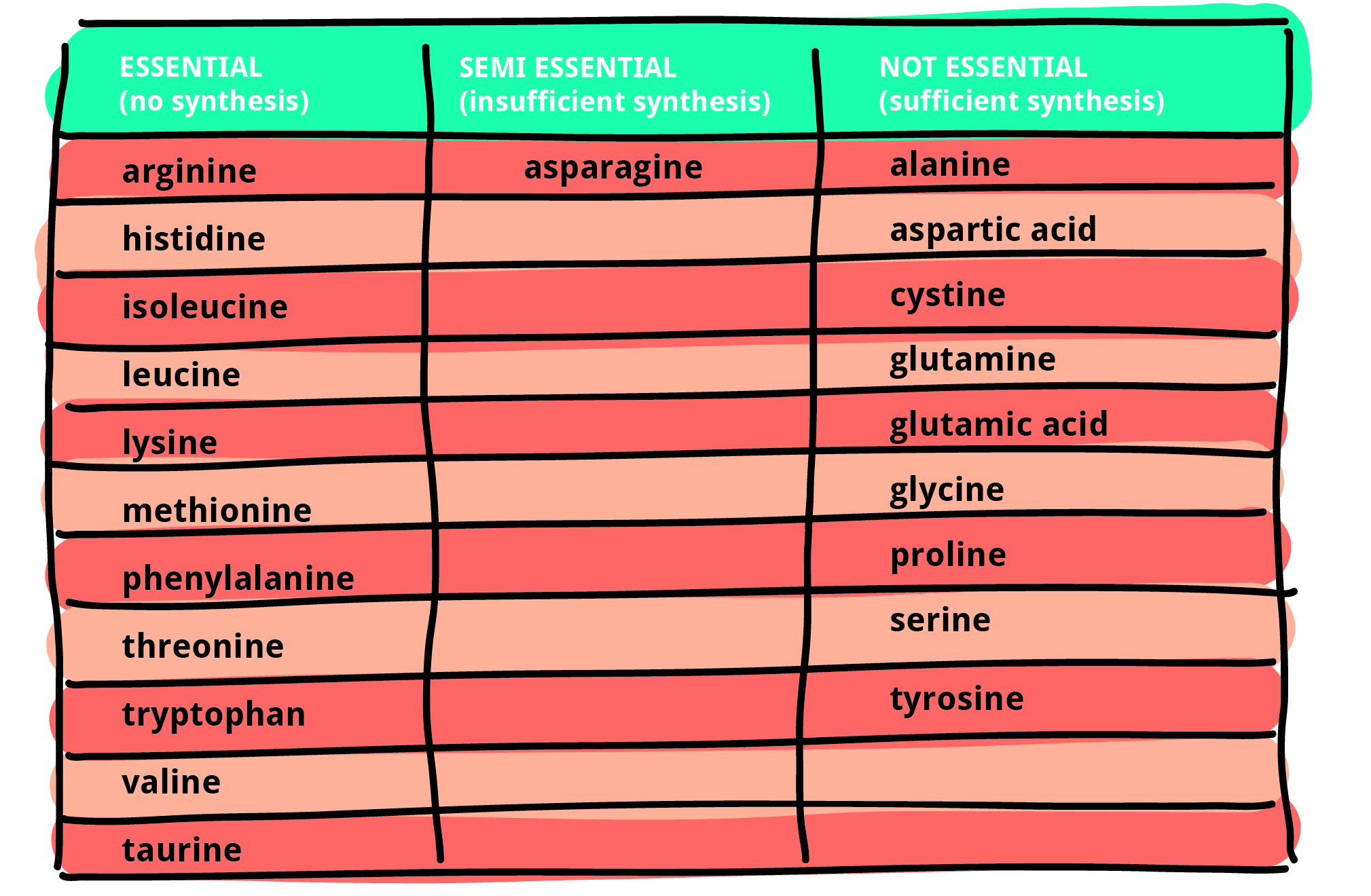
Amino acids can be modified in the body in such a way that bioactive molecules are formed from them. One such amino acid is 3-methylhistidine (3-MH), which is formed by skeletal muscles. 3-MH is released into the blood during muscle breakdown and excreted unchanged in the urine. A previous study reported that plasma 3-MH concentrations were higher in cats with CKD than in healthy controls. This indicates that cats with CKD lose muscle mass. Also, plasma 3-MH levels were elevated in CKD cats with poor appetite compared to cats with normal appetite. This is an indication that muscle mass is reduced when (CKD) cats eat poorly.
Marker of frailty
CreatinineCreatinine is a breakdown product of muscle metabolism. Its blood level is dependent, among many factors, on age, weight, nutritional status and muscle mass. Hence, creatinine levels in the blood vary from individual to individual. Creatinine is continuously excreted in the urine. Increased blood creatinine... in blood plasma is a marker of skeletal muscle mass, but also the main marker of CKD in cats. An increase in creatinine, which is a sign of CKD, can also result from muscle breakdown, as creatinine comes from muscle metabolism. In human medicine, therefore, the concentration of 3-MH is related to that of creatinine (3-MH/crea) in order to have a biomarker for muscle protein metabolism and thus a criterion for frailty in the elderly. In veterinary medicine, the potential relationship between 3-MH concentrations and muscle wasting in cats has not yet been investigated to this point
CKD cats lack essentials
In the recently published study, amino acid concentrations in serum (= plasma, or blood) and faeces were measured to compare amino acid profiles between healthy older cats and cats with CKD.
The authors found that CKD cats have a disturbed serum amino acid profile: CKD cats had lower serum concentrations of three essential (phenylalanine, threonine, tryptophan) and two non-essential amino acids (serine, tyrosine) compared to healthy cats. Changes in serum amino acid profiles occurred in cats with early-stage disease (IRIS stages 1 and 2), and the most severe profile change was found in cats with late-stage CKD (IRIS stages 3 and 4) for several amino acids (aspartic acid, β-alanine, citrulline, leucine, phenylalanine, serine, taurine, tryptophan, valine). In contrast, the authors found no significant differences in the amino acid concentrations in faeces between healthy cats and CKD cats.

This suggests that protein indigestion and subsequent loss of amino acids in faeces may not contribute significantly to the impaired serum amino acid profile in CKD cats. Other possible causes of changes in plasma amino acid status in CKD cats could be inadequate energy intake and increased protein breakdown – in addition to urinary amino acid loss as a result of impaired renal function in CKD.
The majority of the CKD cats in this study had an adequate appetite. However, since a correct calorie calculation was not possible, insufficient protein intake is conceivable as a possible cause for the disturbed amino acid profile of the CKD cats. Finally, the authors found that the 3-MH/Crea ratio did not differ between healthy and older cats with and without muscle loss – unlike in humans.
Several studies – comparable results
A previous study as well as this study showed several comparable differences in amino acid concentrations between healthy and CKD cats. Both studies showed significantly lower concentrations of tryptophan and tyrosine and higher concentrations of citrulline in CKD cats compared to healthy cats.
Serum concentrations of some amino acids were higher in CKD cats than in healthy cats, including the essential amino acid taurine and the non-essential amino acids aspartic acid, citrulline and β-alanine. Circulating concentrations of taurine and citrulline have been found to be higher in cats and humans with renal dysfunction. For citrulline, this finding is attributed to reduced conversion of citrulline to arginine by the kidney. Taurine is an essential amino acid in cats, unlike in humans and dogs, and therefore cats must obtain it from their diet. Higher serum taurine concentrations in CKD cats could be a result of reduced renal excretion. The reason for higher circulating concentrations of β-alanine and aspartic acid in CKD cats is unknown, but is similar to those in humans with kidney disease.
Faecal concentrations of leucine, phenylalanine, lysine, histidine, methionine, tyrosine and tryptophan were dramatically increased in people with end-stage renal disease (ESRD) receiving dialysis compared to healthy controls.
These undigested amino acids in the colon of ESRD patients may favour such gut bacteria that produce the major gut-derived uraemic toxins indoxyl sulphate (IS) and p-cresol sulphate (pCS). In people, cats and dogs with kidney disease, there is an accumulation of indoxyl sulphate and p-cresol sulphate respectively. The accumulation of these uraemic toxins is a consequence of the reduced excretion in chronic kidney disease. This causes the uraemic toxins to build up in the blood.
To dig into the topic, I recommend you read my blog post Uraemic Toxins.
Kidney diet – a faulty compromise
Sufficient supply of high-quality proteins while reducing uraemic toxins, which are naturally produced from proteins, is a challenge in the CKD cat. For this reason, kidney diets are low in protein. However, they are usually not adequately eaten or have such different protein contents that there may be a protein and calorie deficiency. This situation can then lead to muscle loss due to insufficient protein intake. Muscle loss and weight loss lead to weakening of the cat and are the main cause of death in CKD. An adequate amount of high-quality protein is therefore necessary for the CKD cat.
On the other hand, plasma levels of uraemic toxins such as IS and pCS must be drastically reduced. Both uraemic toxins lead to clinical symptoms and progression of kidney disease.
In humans, both uraemic toxins are associated with increased mortality.
To reduce IS and pCS, there is now a special binder that picks up the uraemic precursors produced by the intestinal bacteria in the gut. The precursors are excreted in the faeces together with the binder. This way uraemic toxins can no longer be formed from them. This lowers the plasma level of the uraemic toxins and thus leads out of the dilemma of having to reduce protein in the cat food.
More about the function of so-called OSCAs can also be found in my last blog post.
Bibliography:
- Summers, St. C.; Suchodolski, J.; Quimby, J.; Blake, A.; Keys, D. & Steiner, J.M. (2022): Serum and Fecal Amino Acid Profiles in Cats with Chronic Kidney Disease, Veterinary Science, 9, 84, S. 1–13. . https://doi.org/10.3390/vetsci9020084
-
Goldstein, R.E.; Marks, S.L.; Cowgill, L.D.; Kass, P.H.; Rogers, Q.R. Plasma amino acid profiles in cats with naturally acquired chronic renal failureLoss of kidney function, which may be sudden (acute) or gradual (chronic), as in chronic kidney disease. Loss of kidney function leads to a reduced filtration capacity (glomerular filtration rate) and, thus, an inability to sufficiently filter out urinary substances such as uraemic toxins. Renal.... Am. J. Vet. Res. 1999, 60, 109–113.


